Multiple Choice
Calculate the energy change associated with the transition from 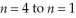 in the hydrogen atom.
in the hydrogen atom.
A) +4.89 × 10-18 J
B) +1.64 × 10-18 J
C) -6.12 × 10-18 J
D) +3.55 × 10-18 J
E) -2.04 × 10-18 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q124: The de Broglie wavelength of an electron
Q125: What are the possible values of n
Q126: How many orbitals are there in the
Q127: What value of l is represented by
Q128: Each of the following sets of quantum
Q129: Give the numbers for m<sub>l</sub> for an
Q130: Which of the following visible colors of
Q132: Define constructive interference.
Q133: Calculate the energy of the violet light
Q134: Describe the shape of a 2p<sub>x</sub> orbital.<br>A)