Multiple Choice
Determine the end (final) value of n in a hydrogen atom transition,if the electron starts in 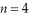 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.
A) 1
B) 5
C) 3
D) 4
E) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q116: Which of the following quantum numbers describes
Q117: Identify the orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt="Identify the
Q118: Identify the orbital. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt="Identify the
Q119: Calculate the frequency of the green light
Q120: _,used to destroy molecules within unwanted cells
Q122: Calculate the wavelength (in nm)of the red
Q123: Calculate the wavelength of a baseball (m
Q124: The de Broglie wavelength of an electron
Q125: What are the possible values of n
Q126: How many orbitals are there in the