Multiple Choice
The complete electron configuration of silicon,element 14,is ________.
A) 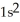
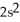
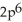 3s23p2
3s23p2
B) 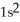
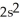 2p10
2p10
C) 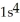
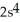 2p6
2p6
D) 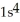 1p62s4
1p62s4
E) 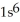 2s62p2
2s62p2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: How many electrons are in the outermost
Q110: Place the following in order of decreasing
Q111: Which of the following statements is TRUE?<br>A)
Q112: Choose the valence orbital diagram that represents
Q113: Identify the transition element.<br>A) Fm<br>B) W<br>C) K<br>D)
Q114: Why is the first ionization energy of
Q116: Give the set of four quantum numbers
Q117: Of the following,which element has the highest
Q118: Below is a list of successive ionization
Q119: Give the ground state electron configuration for