Multiple Choice
The complete electron configuration of titanium,element 22,is ________.
A) 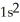
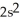
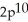
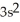 3p6
3p6
B) 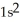
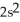
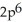
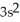
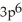 3d24s2
3d24s2
C) 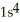
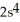
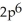
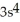 3p4
3p4
D) 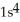
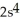
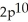 3s4
3s4
E) 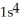
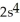
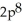
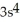 3p2
3p2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q137: Give the number of core electrons for
Q138: Place the following in order of increasing
Q139: How many of the following elements have
Q140: Define ionization energy.
Q141: The element that corresponds to the electron
Q143: Give the ground state electron configuration for
Q144: How many of the following species are
Q145: Give the ground state electron configuration for
Q146: Describe the reaction of the alkali metals
Q147: Why does the size of the transition