Multiple Choice
Which of the following represents the Lewis structure for N?
A) 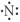
B) 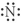
C) 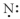
D) 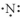
E) 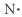
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: Identify the bond with the highest bond
Q123: The electronegativity is 2.1 for H and
Q124: The Lewis structure of sulfuric acid contains<br>A)
Q125: Choose the bond below that is most
Q127: Place the following in order of increasing
Q129: Draw the best Lewis structure for FO<sub>4</sub>⁻
Q130: How many of the following elements can
Q131: Use Lewis theory to determine the chemical
Q132: Identify the weakest bond.<br>A) single covalent bond<br>B)
Q133: Choose the compound below that should have