Multiple Choice
Which of the following represents the Lewis structure for Mg?
A) 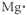
B) 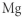
C) 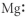
D) 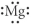
E) 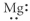
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q62: How many lone pairs are on the
Q76: A triple covalent bond contains<br>A) 0 pairs
Q77: Identify the compound with the smallest percent
Q78: Choose the best Lewis structure for OCl<sub>2</sub>.<br>A)
Q79: Use the bond energies provided to estimate
Q81: Which molecule or compound below contains an
Q82: A reaction is exothermic when<br>A) weak bonds
Q83: Place the following in order of decreasing
Q84: The antimony atom in SbCl<sub>3</sub> would be
Q85: Draw the best Lewis structure for CCl<sub>3</sub><sup>+1</sup>.What