Multiple Choice
Which of the following represents the Lewis structure for S2⁻?
A) 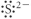
B) 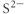
C) 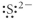
D) 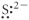
E) 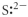
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: Identify the number of bonding pairs and
Q93: Identify the compound with the highest percent
Q94: Using Lewis structures and formal charge,which of
Q95: List the most electronegative atom.
Q96: Give the complete electronic configuration for S<sup>2-</sup>.<br>A)
Q98: Identify the compound with metallic bonding.<br>A) LiI<br>B)
Q99: Give the number of pairs of valence
Q100: Which of the following represents the Lewis
Q101: Choose the bond below that is the
Q102: Choose the bond below that is most