Multiple Choice
Choose the best Lewis structure for SeO42⁻.
A) 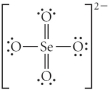
B) 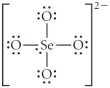
C) 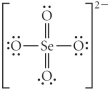
D) 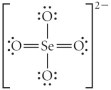
E) 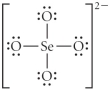
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Rank the following molecules in decreasing bond
Q11: Which of the following represents the Lewis
Q12: Use Lewis theory to determine the chemical
Q13: Identify the bond with the highest bond
Q14: Identify the substance that conducts electricity.<br>A) KBr
Q16: Identify the number of bonding pairs and
Q17: Which of the following processes is endothermic?<br>A)
Q18: Define bond energy.<br>A) energy required to form
Q19: Identify the property of metals that is
Q20: Use the bond energies provided to estimate