Multiple Choice
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 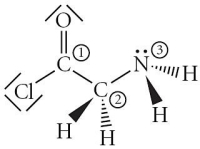
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Q48: Determine the electron geometry (eg)and molecular geometry
Q49: Give the molecular geometry and number of
Q50: A molecule containing a central atom with
Q51: Give the hybridization for the C in
Q52: Identify the hybridization for the carbons in
Q54: Place the following in order of increasing
Q55: Determine the electron geometry (eg)and molecular geometry
Q56: Which of the following statements is TRUE?<br>A)
Q57: Determine the electron geometry (eg),molecular geometry (mg),and
Q58: List the number of sigma bonds and