Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 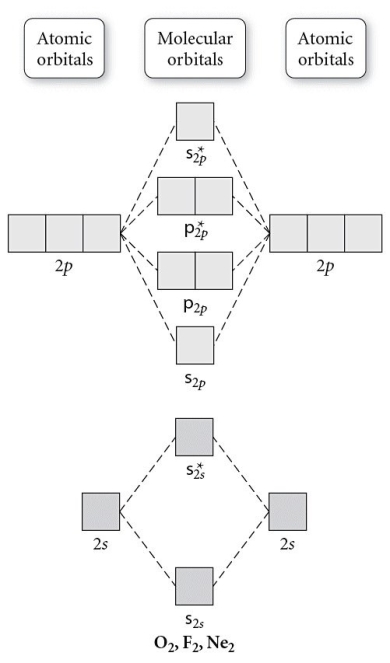
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Using the VSEPR model,the molecular geometry of
Q90: List the number of sigma bonds and
Q91: Give the hybridization for the Br in
Q92: Determine the electron geometry (eg)and molecular geometry
Q93: Determine the electron geometry (eg),molecular geometry (mg),and
Q96: Give the hybridization for the C in
Q97: How many of the following molecules contain
Q98: How many of the following molecules have
Q99: Give the hybridization for the S in
Q100: Give the molecular geometry and number of