Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 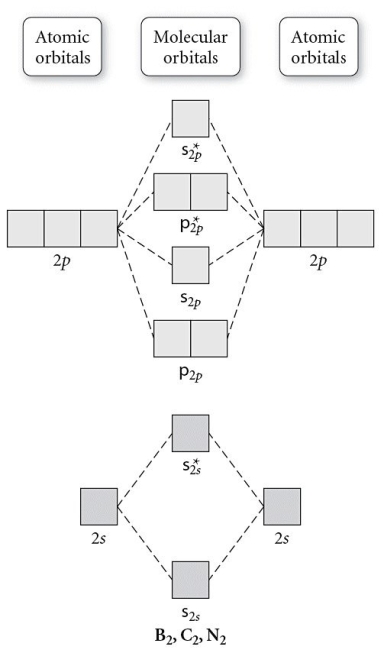
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: If an oil tanker leaks oil in
Q14: Choose the compound below that contains at
Q15: Determine the electron geometry (eg)and molecular geometry
Q16: Identify the number of electron groups around
Q17: Give the approximate bond angle for a
Q19: The VSEPR model predicts the H-N-H bond
Q20: Determine the hybridization about each interior atom
Q21: The bond angle in H<sub>2</sub>Se is<br>A) 107°.<br>B)
Q22: List the number of sigma bonds and
Q23: Describe a sigma bond.<br>A) side by side