Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 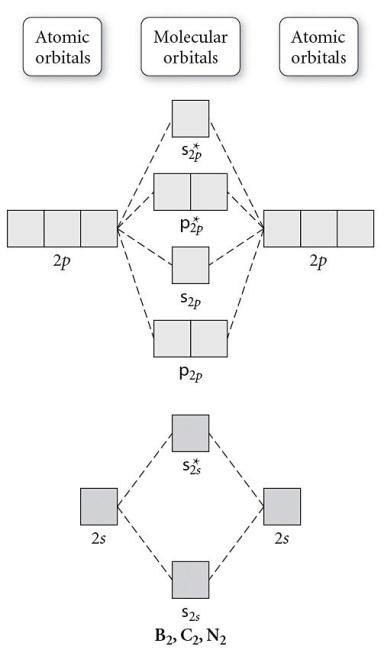
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: Give the electron geometry (eg),molecular geometry (mg),and
Q69: Place the following in order of decreasing
Q70: Give the hybridization for the O in
Q71: Draw the Lewis structure for the molecule
Q72: Identify the number of electron groups around
Q74: Consider the following compound.How many sigma and
Q75: Determine the electron geometry (eg),molecular geometry (mg),and
Q76: Determine the electron geometry (eg)and molecular geometry
Q77: A molecule containing a central atom with
Q78: A molecule,that is sp<sup>3</sup>d<sup>2</sup> hybridized and has