Multiple Choice
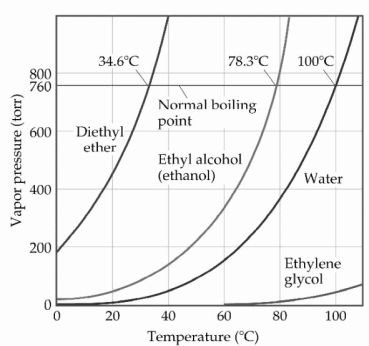 Based on the figure above,the boiling point of water under an external pressure of 0.493 atm is ________°C.
Based on the figure above,the boiling point of water under an external pressure of 0.493 atm is ________°C.
A) 80
B) 40
C) 60
D) 70
E) 90
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q62: What is the strongest type of intermolecular
Q63: Choose the pair of substances that are
Q64: Determine the normal boiling point of a
Q65: Why is the ΔH<sub>vap</sub> higher than ΔH<sub>fus
Q66: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt=" Based on the
Q68: Capillary action occurs because<br>A) cohesive forces are
Q69: What type of intermolecular force causes the
Q70: Which one of the following has a
Q71: The freezing point of water is _.<br>A)
Q72: Identify the compound that has hydrogen bonding.<br>A)