Multiple Choice
The fluorocarbon C2Cl3F3 has a normal boiling point of 47.6°C.The specific heats of C2Cl3F3(l) and C2Cl3F3(g) are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 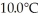 to the gas at 65.0°C is ________ kJ.
to the gas at 65.0°C is ________ kJ.
A) 8.46
B) 1454
C) 29.78
D) 2301
E) 9.63
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Match the following.
Q87: Place the following compounds in order of
Q88: Define condensation.<br>A) A liquid becomes a gas.<br>B)
Q89: Define triple point.<br>A) the temperature, pressure, and
Q90: For automobile engines,identify the best choice of
Q92: Which has the smallest dipole-dipole forces?<br>A) CH<sub>3</sub>CH<sub>2</sub>Cl<br>B)
Q93: Define sublimation.<br>A) the phase transition from solid
Q94: The heat of vaporization of water at
Q95: Place the following substances in order of
Q96: Identify the characteristics of a gas.<br>A) indefinite