Multiple Choice
Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 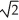 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Identify an element that is NOT in
Q27: When an X-ray beam of l =
Q28: Identify the characteristics of a body-centered cubic
Q29: When two waves interact with the crests
Q30: A common use of polyethylene is<br>A) tire
Q32: What is the edge length of a
Q33: Identify the element with the largest band
Q34: Identify the type of interaction between atoms
Q35: Geologists estimate that _ percent of the
Q36: Identify for the rhombohedral unit cell.<br>A) a