Multiple Choice
Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 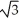 .
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
Correct Answer:

Verified
Correct Answer:
Verified
Q10: NaCl crystallizes in a cubic unit cell
Q68: Identify an element that is added to
Q69: Identify the type of solid for argon.<br>A)
Q70: Identify the element that is the best
Q72: Identify the unit cell that has α
Q75: Which of the following substances should have
Q76: Identify the compound that has the zinc
Q77: A common use of polyethylene terephthalate is<br>A)
Q78: Identify two-dimensional graphite.<br>A) buckyball<br>B) diamond<br>C) graphene<br>D) fullerene<br>E)
Q114: Nickel has a face-centered cubic structure and