Multiple Choice
Express the equilibrium constant for the following reaction. Pb(NO3) 2(aq) + 2 NaI(aq) ⇌ PbI2(s) + 2 NaNO3(aq)
A) K = 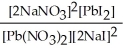
B) K = 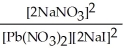
C) K = 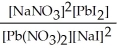
D) K = 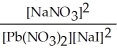
E) K = 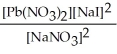
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The equilibrium constant is given for one
Q13: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q14: Which of the following statements is TRUE?<br>A)
Q15: What is Δn for the following equation
Q16: Define dynamic equilibrium.<br>A) no reactants react<br>B) no
Q18: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q19: Consider the following reaction at equilibrium.What effect
Q20: Consider the following reaction at equilibrium.What effect
Q21: Consider the following reaction: CuS(s)+ O<sub>2</sub>(g)⇌ Cu(s)+
Q22: In a reaction mixture containing only products,what