Multiple Choice
Express the equilibrium constant for the following reaction. 4 CH3Cl(g) + 2 Cl2(g) ⇔ 4 CH2Cl2(g) + 2 H2(g)
A) K = 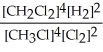
B) K = 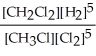
C) K = 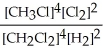
D) K = 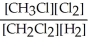
E) K = 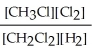
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Consider the following reaction at equilibrium.What will
Q37: At a certain temperature,bromine and nitric oxide
Q38: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q39: What is Δn for the following equation
Q40: Dinitrogen tetroxide partially decomposes according to the
Q42: Define Le Chatelier's Principle.
Q43: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q44: Calculate P [NO]<sub>eq</sub>,if P [NOCl]<sub>eq</sub> = 0.33
Q45: Consider the following reaction at equilibrium.What effect
Q46: Determine the value of K<sub>c</sub> for the