Multiple Choice
Express the reverse equilibrium constant for the following reaction. 4 CH3Cl(g) + 2 Cl2(g) ⇔ 4 CH2Cl2(g) + 2 H2(g)
A) K = 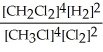
B) K = 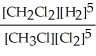
C) K = 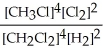
D) K = 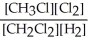
E) K = 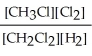
Correct Answer:

Verified
Correct Answer:
Verified
Q92: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q93: Consider the following reaction: COCl<sub>2</sub>(g)⇌ CO(g)+ Cl<sub>2</sub>(g)<br>A
Q94: What is △n for the following equation
Q95: Define Le Chatelier's Principle.<br>A) When a chemical
Q96: Consider the following reaction at equilibrium.What will
Q99: Which of the following statements is TRUE?<br>A)
Q100: Consider the following reaction: Xe(g)+ 2 F<sub>2</sub>(g)⇌
Q101: The equilibrium constant is given for two
Q102: What is Δn for the following equation
Q119: The equilibrium constant is equal to 5.00