Multiple Choice
Express the equilibrium constant for the following reaction. 10 PCl5(g) ⇌ 10 PCl3(g) + 10 Cl2(g)
A) K = 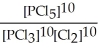
B) K = 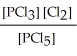
C) K = 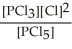
D) K = 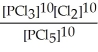
E) K = 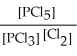
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q127: What is Δn for the following equation
Q128: Consider the following reaction at equilibrium.What effect
Q129: In which of the following reactions will
Q130: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q131: K<sub>c</sub> is 1.67 × 10<sup>20</sup> at 25°C
Q133: Consider the following reaction at equilibrium.What effect
Q134: The following reaction is exothermic.Which change will
Q135: Express the equilibrium constant for the following
Q136: Express the equilibrium constant for the following
Q137: Express the equilibrium constant for the following