Multiple Choice
Express the reverse equilibrium constant for the following reaction. 8 H2(g) + 8 Br2(g) ⇌ 16 HBr(g)
A) K = 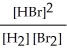
B) K = 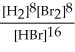
C) K = 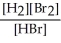
D) K = 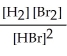
E) K = 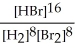
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: In a reaction mixture containing only products,what
Q23: Consider the following reaction and its equilibrium
Q24: Give the direction of the reaction,if K
Q24: Give the direction of the reaction,if K
Q25: Consider the following reaction: CO<sub>2</sub>(g)+ C(graphite)⇌ 2
Q28: The equilibrium constant is given for two
Q29: What is △n for the following equation
Q30: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q31: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q32: How is the reaction quotient different from