Multiple Choice
Express the equilibrium constant for the following reaction. 20 CH3Br(g) + 10 Br2(g) ⇔ 20 CH2Br2(g) + 10 H2(g)
A) K = 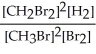
B) K = 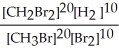
C) K = 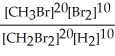
D) K = 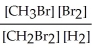
E) K = 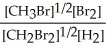
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Consider the following reaction at equilibrium.What effect
Q67: In a reaction mixture containing reactants and
Q68: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q69: Consider the following reaction at equilibrium.What will
Q70: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>
Q72: The following reaction is exothermic.Which change will
Q73: The equilibrium constant is given for one
Q74: Determine the value of K<sub>c</sub> for the
Q75: What is Δn for the following equation
Q76: Express the equilibrium constant for the following