Multiple Choice
The Kp for the reaction below is 1.49 × 108 at 100.0°C: CO(g) + 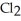 (g) ⇌
(g) ⇌ 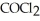 (g)
(g)
In an equilibrium mixture of the three gases, 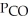 =
= 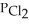 = 2.22 × 10-4 atm.The partial pressure of the product,phosgene (
= 2.22 × 10-4 atm.The partial pressure of the product,phosgene ( 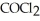 ) ,is ________ atm.
) ,is ________ atm.
A) 7.34
B) 3.02 × 1015
C) 3.31 × 10-16
D) 3.31 × 104
E) 6.67 × 1011
Correct Answer:

Verified
Correct Answer:
Verified
Q100: Consider the following reaction: Xe(g)+ 2 F<sub>2</sub>(g)⇌
Q101: The equilibrium constant is given for two
Q102: What is Δn for the following equation
Q103: Calculate the value of [N<sub>2</sub>]<sub>eq</sub> if [H<sub>2</sub>]<sub>eq</sub>
Q104: Consider the following reaction and its equilibrium
Q106: Consider the following reaction at equilibrium.What effect
Q107: Consider the following reaction: NO(g)+ SO<sub>3</sub>(g)⇌ NO<sub>2</sub>(g)+
Q108: In which of the following reactions will
Q109: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q110: Express the equilibrium constant for the following