Multiple Choice
At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345. 2 SO2(g) + O2(g) ⇌ 2 SO3(g)
At equilibrium,the partial pressure of SO2 is 35.0 atm and that of 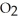 is 15.9 atm.The partial pressure of SO3 is ________ atm.
is 15.9 atm.The partial pressure of SO3 is ________ atm.
A) 82.0
B) 4.21 × 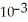
C) 192
D) 6.20 × 10-4
E) 40.2
Correct Answer:

Verified
Correct Answer:
Verified
Q80: How will equilibrium be affected,with the same
Q81: Match the following.
Q82: Consider the following reaction at equilibrium.What will
Q83: Consider the following reaction at equilibrium.What effect
Q84: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)⇌
Q86: Identify the change that will always shift
Q87: For which reaction will K<sub>p</sub> = K<sub>c</sub>?<br>A)
Q88: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q89: Why aren't solids or liquids included in
Q90: Consider the following reaction and its equilibrium