Multiple Choice
What is the hydroxide ion concentration and the pH for a hydrochloric acid solution that has a hydronium ion concentration of 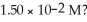
A) 6.67 × 10-12 M, 2.82
B) 6.67 × 10-12 M, 11.18
C) 6.67 × 10-13 M, 1.82
D) 6.67 × 10-13 M, 12.17
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: _ is a painful burning sensation in
Q66: Determine the pOH in a 0.235 M
Q67: Determine the pH of a 0.22 M
Q68: Calculate the pH of a 1.60 M
Q69: Which of the following is a Br∅nsted-Lowry
Q71: Identify the products when hydrochloric acid completely
Q72: Calculate the pH of a solution that
Q73: Which of the following is a weak
Q74: Acid reflux is when _ backs up
Q75: Which of the following is NOT a