Multiple Choice
The pH of an aqueous solution at 25.0°C is 10.66.What is the molarity of 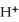 in this solution?
in this solution?
A) 2.2 × 10-11
B) 4.6 × 10-4
C) 3.3
D) 1.1 × 10-13
E) 4.6 × 1010
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Identify the acid that is used in
Q37: Determine the [H<sub>3</sub>O<sup>+</sup>] concentration for a 0.100
Q38: Calculate the pOH in an aqueous solution
Q40: Which one of the following salts,when dissolved
Q41: Calculate the molarity of hydroxide ion in
Q42: What is the pH of a 0.0040
Q43: Identify the weak monoprotic acid.<br>A) H<sub>2</sub>CO<sub>3</sub><br>B) NaOH<br>C)
Q44: Identify the strongest acid.<br>A) HFO<sub>4</sub><br>B) HFO<sub>3</sub><br>C) HFO<sub>2</sub><br>D)
Q45: Determine the K<sub>a</sub> of an acid whose
Q46: Which of the following species is amphoteric?<br>A)