Multiple Choice
A 7.0 × 10-3 M aqueous solution of 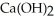 at 25.0°C has a pH of ________.
at 25.0°C has a pH of ________.
A) 12.15
B) 1.85
C) 1.4 × 10-2
D) 7.1 × 10-13
E) 11.85
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q102: Identify the products that are in equilibrium
Q104: Calculate the pH for an aqueous hydrobromic
Q105: Determine the pH of a 0.227 M
Q106: Describe the relationship between molecular structure and
Q108: Determine the pOH of a 0.188 M
Q109: Calculate the pOH of a nitrous acid
Q110: Place the following in order of increasing
Q111: Identify the food that does NOT irritate
Q112: Calculate the pH of a solution that
Q186: What is the pH of a 0.020