Multiple Choice
A solution is prepared by dissolving 0.23 mol of hydrazoic acid and 0.27 mol of sodium azide in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The Ka of hydrazoic acid is 1.9 × 10-5.
A) 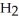 O
O
B) 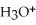
C) azide
D) hydrazoic acid
E) This is a buffer solution: the pH does not change upon addition of acid or base.
Correct Answer:

Verified
Correct Answer:
Verified
Q105: Determine the molar solubility of MgCO<sub>3</sub><sub> </sub>in
Q106: Give the equation for a supersaturated solution
Q107: What is the pH of the resulting
Q108: Identify the most common indicator.<br>A) alizarin<br>B) thymol
Q109: What is the pH of a solution
Q111: A sample contains MgNH<sub>4</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>Sb<sub>2</sub>S<sub>3</sub>,KBr,Cr(OH)<sub>3</sub>,and PbCl<sub>2</sub>.Identify the
Q112: Define buffer capacity.<br>A) Buffer capacity is the
Q113: Describe what happens at low pH for
Q114: Identify the ion that is less soluble
Q115: The molar solubility of Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is 8.89