Multiple Choice
What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 50.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for 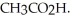
A) 8.26
B) 9.26
C) 11.13
D) 12.40
Correct Answer:

Verified
Correct Answer:
Verified
Q126: Consider the K<sub>sp</sub> values for two compounds:
Q127: Which of the following solutions is a
Q128: Identify a good buffer.<br>A) small amounts of
Q129: Give the expression for the solubility product
Q130: Identify the structure for antifreeze.<br>A) CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub><br>B) CH<sub>3</sub>OH<br>C)
Q132: What is the pH of a buffer
Q133: If the pKa of HCHO<sub>2</sub> is 3.74
Q134: A buffer solution is 0.100 M in
Q135: A solution containing CaCl<sub>2</sub> is mixed with
Q136: Explain the common ion effect with respect