Multiple Choice
A solution of NaF is added dropwise to a solution that is 0.0144 M in Ba2+.When the concentration of 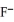 exceeds ________ M,BaF2 will precipitate.Neglect volume changes.For BaF2,Ksp = 1.7 × 10-6.
exceeds ________ M,BaF2 will precipitate.Neglect volume changes.For BaF2,Ksp = 1.7 × 10-6.
A) 5.9 × 10-5
B) 1.1 × 10-2
C) 2.4 × 10-8
D) 2.7 × 10-3
E) 1.2 × 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Calculate the molar solubility of thallium chloride
Q25: When titrating a weak base with a
Q26: What is the hydronium ion concentration in
Q27: A 500.0 mL sample of 0.18 M
Q28: A solution contains 3.8 × 10<sup>-2</sup><sup> </sup>M
Q30: Identify the salts that are in hard
Q31: In which of the following solutions would
Q32: A 200.0 mL sample of 0.18 M
Q33: Determine the molar solubility of AgI in
Q34: A sample contains MgNH<sub>4</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>Sb<sub>2</sub>S<sub>3</sub>,KBr,Cr(OH)<sub>3</sub>,and PbCl<sub>2</sub>.Identify the