Multiple Choice
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten 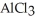 with an electrical current of 12.0 A?
with an electrical current of 12.0 A?
A) 27.0
B) 9.00
C) 1.19 × 103
D) 2.90 × 105
E) 3.57 × 103
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: Identify the characteristics of a spontaneous reaction.<br>A)
Q104: Balance the following redox reaction if it
Q105: The standard emf for the cell using
Q106: What is undergoing reduction in the redox
Q107: Which of the following is the strongest
Q109: Which of the following metals will dissolve
Q110: What element is being reduced in the
Q111: Identify the location of reduction in an
Q112: Which of the following is the weakest
Q113: Calculate the cell potential for the following