Multiple Choice
Give the product of the oxidation of the following compound. 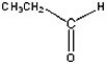
A) CH3CH2CH2CHO
B) CH3CH2CH2OH
C) CH3COOCH3
D) CH3CH2COOH
E) CH3CH2OCH3
Correct Answer:

Verified
Correct Answer:
Verified
Q94: How many isomers can be drawn for
Q95: Write a balanced chemical equation to represent
Q96: A carboxylic acid reacts with itself to
Q97: Give the organic product for the following
Q98: Give the organic product for the following
Q99: Name the following compound. CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>A) ethyl propyl
Q100: Name the following compound. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt="Name
Q102: Write the balanced chemical equation that represents
Q103: Which of the following compounds is an
Q104: Identify the most oxidized compound.<br>A) CH<sub>3</sub>CH<sub>2</sub>COOH<br>B) CH<sub>3</sub>CH<sub>2</sub>CHO<br>C)