Multiple Choice
Which of the following atoms contains the largest number of neutrons?
A) 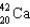
B) 
C) 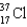
D) 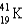
E) 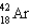
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: The molar mass of platinum is
Q54: A 1.583 g sample of an
Q55: What is the mass number of an
Q56: Sodium sulfate has the chemical formula Na<sub>2</sub>SO<sub>4</sub>.Based
Q58: Copper has an atomic weight of 63.55
Q59: The elements in group 2A are known
Q60: What is the empirical formula of an
Q61: What is the name of the halogen
Q62: Which atom is most likely to form
Q99: Millikan's oil drop experiment determined the charge