Multiple Choice
The mass spectrum of an element with two naturally occurring isotopes is shown below.What is the best estimate of the element's (average) atomic weight? 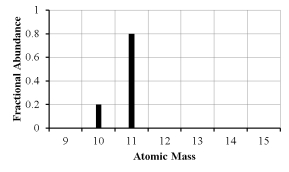
A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q81: What is the mass of 8.04
Q82: What is the symbol for an ion
Q83: A neutral atom of the isotope <sup>197</sup>Au
Q84: Which element is most likely to form
Q85: In reactions,metals generally lose electrons to become
Q87: A sample of an element consists of
Q88: What halogen is in the third period?<br>A)
Q89: What is the common name for PH<sub>3</sub>?<br>A)
Q90: Which of the following elements is not
Q91: What is the correct name for NH<sub>4</sub>NO<sub>3</sub>?<br>A)