Multiple Choice
The products of the complete combustion of octane,C8H18,are carbon dioxide and water.Write a balanced chemical equation for this reaction.
A) C8H18( 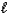 ) 8 C(s) + 9 H2(g)
) 8 C(s) + 9 H2(g)
B) C8H18( 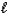 ) + 25 O2(g) 8 CO2(g) + 9 H2O(g)
) + 25 O2(g) 8 CO2(g) + 9 H2O(g)
C) 2 C8H18( 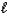 ) + 25 O2(g) 16 CO2(g) + 18 H2O(g)
) + 25 O2(g) 16 CO2(g) + 18 H2O(g)
D) C8H18( 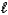 ) + 16 O2(g) 8 CO2(g) + 9 H2(g)
) + 16 O2(g) 8 CO2(g) + 9 H2(g)
E) 2 C8H18( 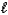 ) + 17 O2(g) 16 CO(g) + 18 H2O(g)
) + 17 O2(g) 16 CO(g) + 18 H2O(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q33: What are the smallest whole number
Q35: What are the spectator ions in the
Q36: What is the net ionic equation
Q37: Which of the following is a
Q39: Which anion will form a precipitate with
Q40: Which equation best represents the balanced
Q41: When solutions of barium chloride and
Q42: The net ionic equation for the
Q43: The complete combustion of nonane,C<sub>9</sub>H<sub>20</sub>,yields carbon
Q72: Which of the following compounds is a