Multiple Choice
The net ionic equation for the reaction between aqueous ammonia and hydrochloric acid is
A) HCl(aq) + NH3(aq) NH4Cl(aq) .
B) H+(aq) + OH-(aq) H2O( 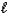 ) .
) .
C) HCl(aq) + OH-(aq) Cl-(aq) + H2O( 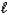 ) .
) .
D) H+(aq) + NH3(aq) NH4+(aq) .
E) H+(aq) + Cl-(aq) + NH3(aq) NH4+(aq) + Cl-(aq) .
Correct Answer:

Verified
Correct Answer:
Verified
Q5: If an aqueous solution of _ is
Q18: Which of the following elements generally acts
Q32: All of the following compounds are insoluble
Q64: Which of the following would
Q65: Which of the following statements is/are CORRECT?<br>1)A
Q66: Which anion will form a precipitate with
Q67: Which one of the following equations
Q68: Which of the following compounds is
Q71: Which of the following is a
Q72: The <span class="ql-formula" data-value="\underline{\text{ net