Multiple Choice
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced?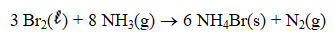
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: An aqueous nitric acid solution has a
Q35: One step in the isolation of pure
Q36: What mass of Na<sub>2</sub>CO<sub>3</sub> is present in
Q37: How many grams of dioxygen are required
Q38: Zn reacts with hydrochloric acid.<br>Zn(s)+ 2
Q40: How many moles of Mg<sub>3</sub>P<sub>2</sub>(s)can be
Q41: A certain compound has a molar mass
Q42: If the complete combustion of an
Q43: Calculate the number of moles of O<sub>2</sub>
Q44: PCl<sub>3</sub> can be produced from the