Multiple Choice
Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride
(M = 102.1 g/mol) .C7H6O3(s) + C4H6O3( 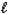 ) C9H8O4(s) + C2H4O2(
) C9H8O4(s) + C2H4O2( 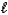
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
Correct Answer:

Verified
Correct Answer:
Verified
Q9: An unknown diprotic acid (H<sub>2</sub>A)requires 44.39 mL
Q10: Complete combustion of a 0.30-mol sample of
Q11: Under certain conditions the reaction of
Q12: Transmittance,T,is defined as the intensity of light
Q13: What volume of 2.52 M HCl
Q15: Aspirin is produced by the reaction
Q16: Magnesium reacts with iodine gas at high
Q17: Of the following,the only empirical formula is<br>A)
Q18: Sulfur dioxide will react with water
Q18: The percent yield of a chemical reaction