Multiple Choice
If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq) + 2 HCl(aq) 2 NaCl(aq) + H2O( 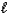 ) + CO2(g)
) + CO2(g)
A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Soft drink bottles are made of polyethylene
Q30: The reaction of 5.07 g N<sub>2</sub> with
Q64: A 2.841 g sample of a hydrocarbon
Q65: Nitric oxide is made from the
Q67: How many moles of sodium bromide
Q68: In combustion analysis,the gases produced by the
Q70: Chlorine was passed over 2.02 g of
Q71: Polyethylene is a polymer consisting of only
Q72: The complete combustion of 0.32 moles of
Q74: Consider the fermentation reaction of glucose:<br>C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>