Multiple Choice
Determine the standard enthalpy of formation of Fe2O3(s) given the thermochemical equations below.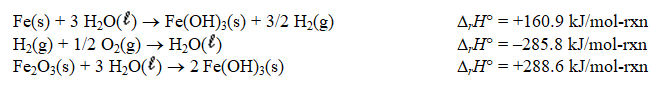
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: Internal energy and enthalpy are state functions.What
Q44: Which of the following processes will result
Q46: Calculate the energy in the form
Q47: Determine the enthalpy change for the
Q48: A 170.0-g sample of metal at 79.00°C
Q50: What is the standard enthalpy of
Q51: What is the minimum mass of
Q52: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q53: A 100 g sample of each
Q54: How much energy is needed to convert