Multiple Choice
Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid calcium nitrate?
A) Ca2+(aq) + 2NO3-(aq) Ca(NO3) 2(s)
B) Ca(OH) 2(s) + 2HNO3(aq) Ca(NO3) 2(s) + 2H2O( 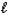 )
)
C) Ca(s) + N2(g) + 3O2(g) Ca(NO3) 2(s)
D) Ca(s) + 2HNO3(aq) Ca(NO3) 2(s) + H2(g)
E) Ca(s) + 2N(g) + 6O(g) Ca(NO3) 2(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The thermochemical equation for the combustion
Q15: The overall chemical equation resulting from
Q16: Which of the following statements is/are CORRECT?<br>1)Specific
Q17: What is the change in internal
Q18: Calculate the energy in the form
Q20: Determine the standard enthalpy of formation of
Q21: If 35.0 g H<sub>2</sub>O at 22.7
Q22: When 10.0 g KOH is dissolved
Q24: If 46.1 g Cu at 11.6
Q61: In thermodynamics,a(n)_ is defined as the object,or