Multiple Choice
The electron in a hydrogen atom,originally in level 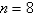 ,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? (
,undergoes a transition to a lower level by emitting a photon of wavelength 3745 nm.What is the final level of the electron? ( 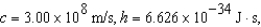
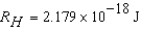 )
)
A) 5
B) 6
C) 8
D) 9
E) 1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: The difference in energy between adjacent energy
Q41: According to Heisenberg's _ principle,it is impossible
Q44: Which of the following is/are correct postulates
Q46: Which of the following ranks regions of
Q47: Which of the following statements is/are CORRECT?<br>1)FM
Q47: The size of an electron orbital is
Q50: If the de Broglie wavelength of
Q52: What is the wavelength of a photon
Q53: Which of the following statements concerning quantum
Q54: A device emits light at 761.7