Multiple Choice
How many orbitals have the following set of quantum numbers: n = 5, 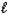 = 1,
= 1, 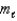 = -1?
= -1?
A) 0
B) 1
C) 3
D) 6
E) 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which of the following regions of the
Q33: Which type of experiment demonstrates that an
Q45: The _ of a photon of light
Q63: What is the energy of a photon
Q64: What is the de Broglie wavelength of
Q66: Which of the following orbital boundary surfaces
Q67: What is the value of the orbital
Q70: What is the de Broglie wavelength of
Q71: According to experiments concerned with the photoelectric
Q72: Which of the following properties is associated