Multiple Choice
Which of the following properties is associated with the value of the 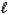 quantum number?
quantum number?
A) the shape of an orbital
B) the size of an orbital
C) the number of electrons in an orbital
D) the energy of an orbital
E) the orientation in space of an orbital
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: A possible value of the magnetic quantum
Q22: Erwin Schrödinger developed a model for the
Q23: The energy required to break one mole
Q24: The Bohr model predicts that the energy
Q28: How many values are there for the
Q29: A 4p orbital has ?<br>A) 3 planar
Q38: How many p orbitals are in the
Q56: A point in a standing wave that
Q57: Occupied states or energy levels in the
Q63: For which of the following transitions would