Multiple Choice
Which of the following statements is/are CORRECT?
1) The angular momentum quantum number, 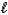
,of an electron in an s orbital is always equal to 0.
2) The magnetic quantum number, 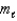
,of an electron in a p orbital is +1,0,or -1.
3) The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following statements is INCORRECT?<br>A)
Q19: Which type of experiment demonstrates that light
Q29: In Bohr's atomic theory,when an electron moves
Q31: A red laser pointer emits light
Q32: According to the Bohr model for the
Q37: A device operates at a frequency of
Q38: Which of the following sets of quantum
Q39: What is the wavelength of light emitted
Q40: Which of the following statements is/are CORRECT?<br>1)A
Q41: A light emitting diode (L.E.D.)emits photons with