Multiple Choice
Which of the following sets of quantum numbers (n,l,ml, ms) is not permissible?
A) 3 3 -3 + 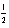
B) 2 1 -1 + 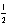
C) 2 0 0 + 
D) 2 1 0 + 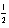
E) 4 2 -1 - 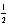
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: What is the wavelength of a
Q12: What is the total number of orbitals
Q14: Which of the following statements is/are CORRECT?<br>1)A
Q15: The n = _ shell is the
Q18: What is the frequency of gamma
Q19: What is the total number of orbitals
Q20: A possible value of the magnetic quantum
Q36: The contribution for which de Broglie is
Q58: What evidence does the photoelectric effect provide
Q63: For which of the following transitions would