Short Answer
The ________ quantum number is given the symbol 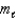
.This quantum number is related to the orientation in space of an orbital.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: For a proton (mass = 1.673
Q3: What is the binding energy of
Q5: Which of the following orbital boundary surfaces
Q6: What is the energy of a photon
Q7: For which of the following transitions would
Q7: Which of the following is a representation
Q8: A _,designated by the Greek symbol
Q9: What is the energy per mole of
Q10: Which type of orbital is designated n
Q11: What is the wavelength of a