Multiple Choice
What 2+ ion has the following ground state electron configuration? 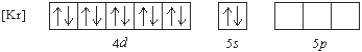
A) Cd2+
B) Sr2+
C) Zn2+
D) Sn2+
E) None
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which of the following electron configurations is
Q6: Which of the following electron configurations corresponds
Q10: What noble gas core precedes the valence
Q11: What is the ground state electron configuration
Q12: A metal nitride forms when sodium reacts
Q13: Rank the following atoms in order decreasing
Q14: A metal oxide forms when strontium reacts
Q29: An atom of which of the following
Q65: Explain why the first ionization energy for
Q79: Place the following ions in order from