Multiple Choice
The ground-state electron configuration of a 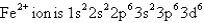 .Therefore,
.Therefore, 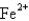 Is
Is
A) paramagnetic with four unpaired electrons.
B) diamagnetic.
C) paramagnetic with one unpaired electron.
D) paramagnetic with three unpaired electrons.
E) paramagnetic with two unpaired electrons.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: How many electrons can be described by
Q38: What is a possible set of quantum
Q40: How many unpaired electrons are found in
Q41: What is the maximum number of electrons
Q43: Which of the following orbital diagrams
Q44: Which ground-state electron configuration is
Q45: How many electrons can be described by
Q46: Rank the following ions in order of
Q47: Which of the following statements concerning the
Q60: An atom of which of the following