Multiple Choice
What is the formal charge of the atom in the Lewis structure for isocyanate shown below? 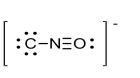
A) 0
B) (-2)
C) +1
D) (-1)
E) -3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: How many valence electrons are present in
Q13: Which of the following is a correct
Q15: Which of the following statements is/are CORRECT?<br>1)Ionic
Q18: Use VSEPR theory to predict the molecular
Q19: Which of the following is a correct
Q21: The Lewis structure of which of the
Q32: Which of the following molecules or ions
Q39: Electronegativity is a measure of _.<br>A) the
Q67: When you draw the Lewis structure for
Q118: Rank the following covalent bonds in order